Post Market Surveillance Plan Template
Post Market Surveillance Plan Template - Here are some of the images for Post Market Surveillance Plan Template that we found in our website database, related for April Free Calendar, Burlap Advent Calendar, Caddo Parish Court Date, Free Podcast Agreement Template, Amazon Plan Of Action Template, Hotel Forecast Template, Start Of An Old Boast Crossword, Edible Lady Fingers Crossword Clue, New Hire Checklist Template Excel, Cell Membrane Coloring Worksheet Key, Dutch Craze Of 1636 Crossword Clue, Feline That May Be Feral Crossword, What Should I Wear To Court Female, Church Notes Template, Winter Hrs In Chicago Crossword, B2b Content Marketing Strategy Template, Pindar Product Crossword Clue, Birth Certificate From El Salvador, Clerics Closetful Nyt Crossword Clue, Alphabet Free Printable Worksheets,

Post Market Surveillance Plan Template QualityMedDev

Post Market Surveillance Plan Template

Post Market Surveillance Plan PMS Plan Template

Post market surveillance plans: How to write one for CE Marking
Output of the postmarket surveillance (PMS) plan Colour figure can be

Post market surveillance is in itself a monitoring and measuring

Post Market Surveillance Report ISO 13485 templates MDCG 2020 8

ISO 20416:2020 Post Market Surveillance Medical Device Manufacturers

(PDF) EU postmarket surveillance plans for medical devices
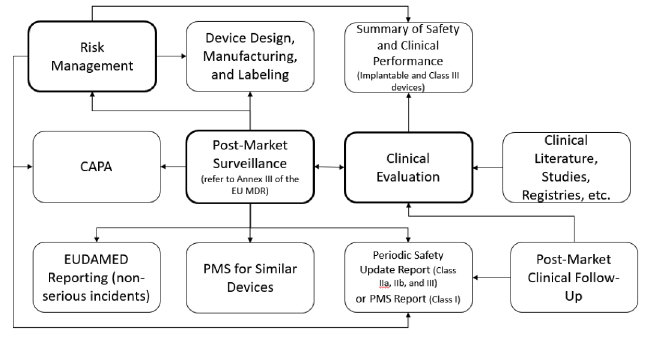
Column EU MDR Post Market Surveillance: Active Integrated Risk
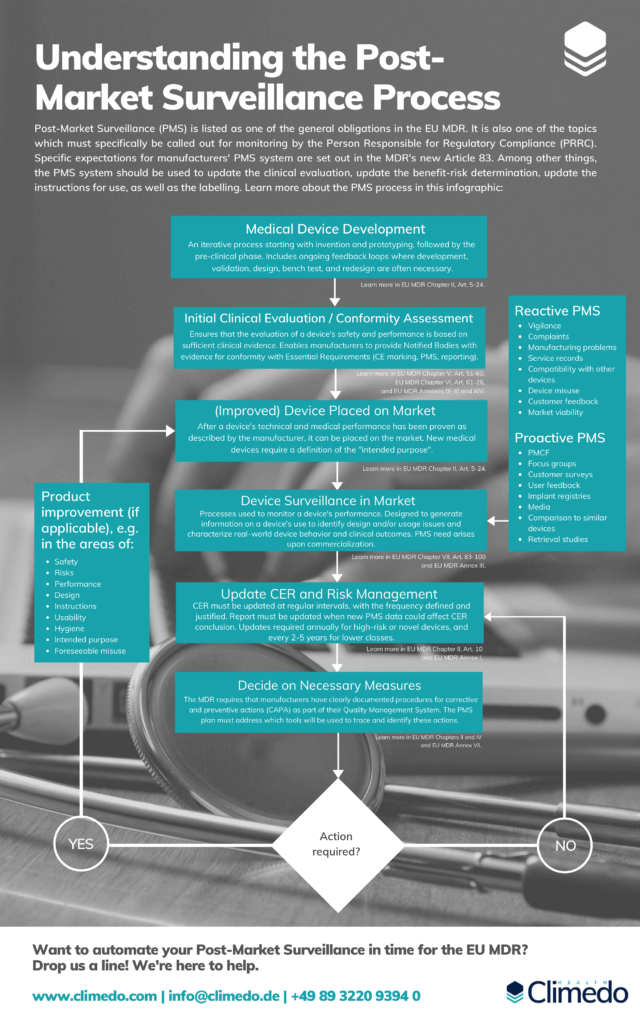
Getting your Post Market Surveillance up to Speed with the EU MDR

Post Market Surveillance Procedure

Post Market Surveillance Plan Template
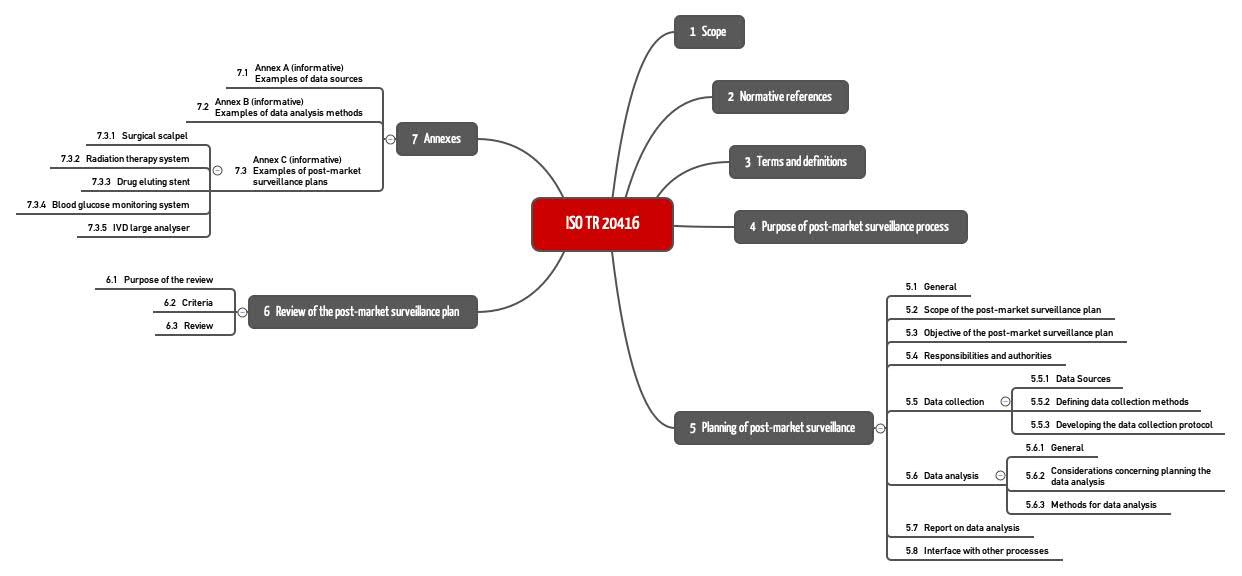
Post Market Surveillance Plan konform ISO 20416: 7 typische Fehler
Post Market Surveillance (PMS) for Medical Devices SimplerQMS
.png)
Understanding Post Market Surveillance Requirements for Medical Devices

The Definitive Guideline on Post Market Surveillance (PMS) Activities
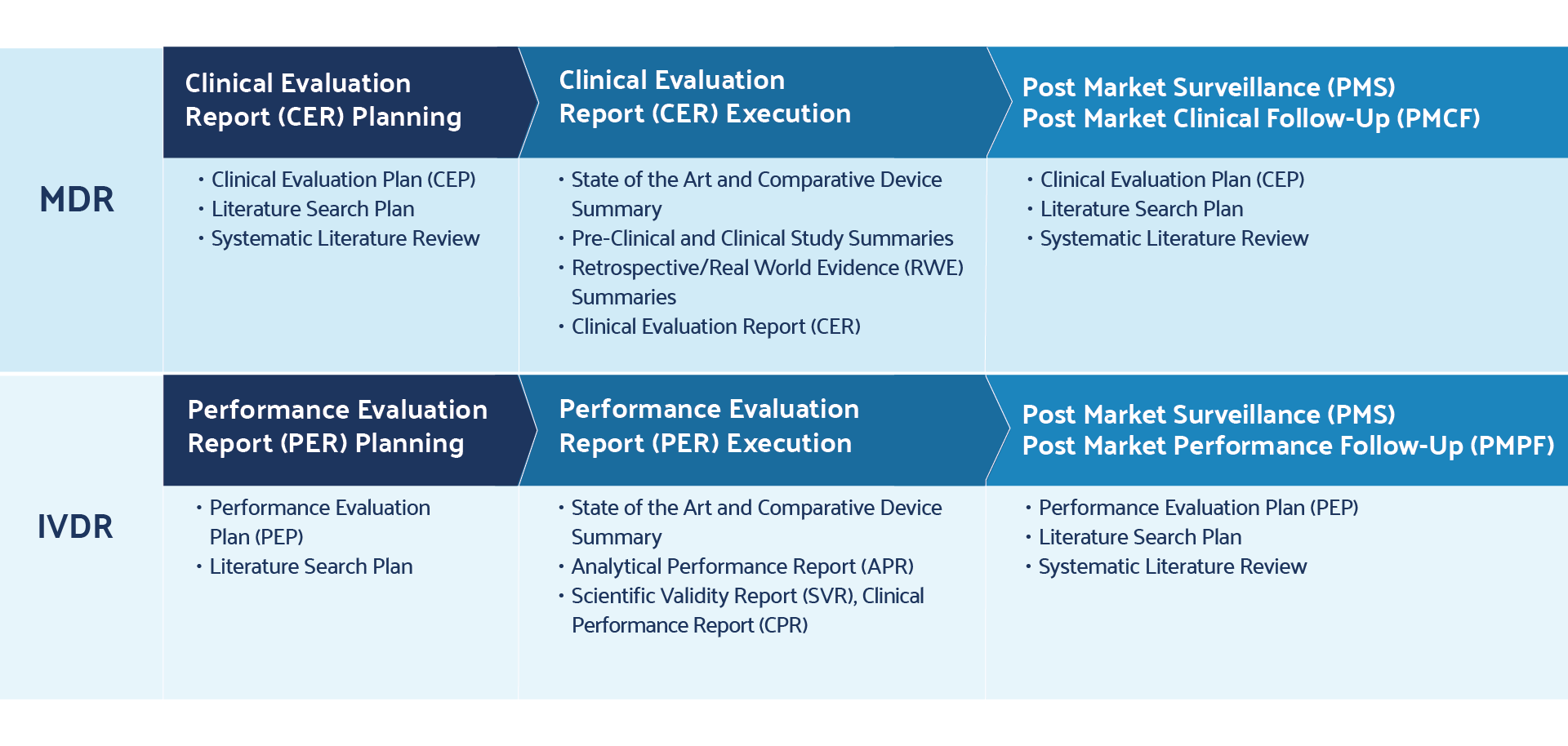
Literature Review Best Practices Accelerate EU MDR Post Market
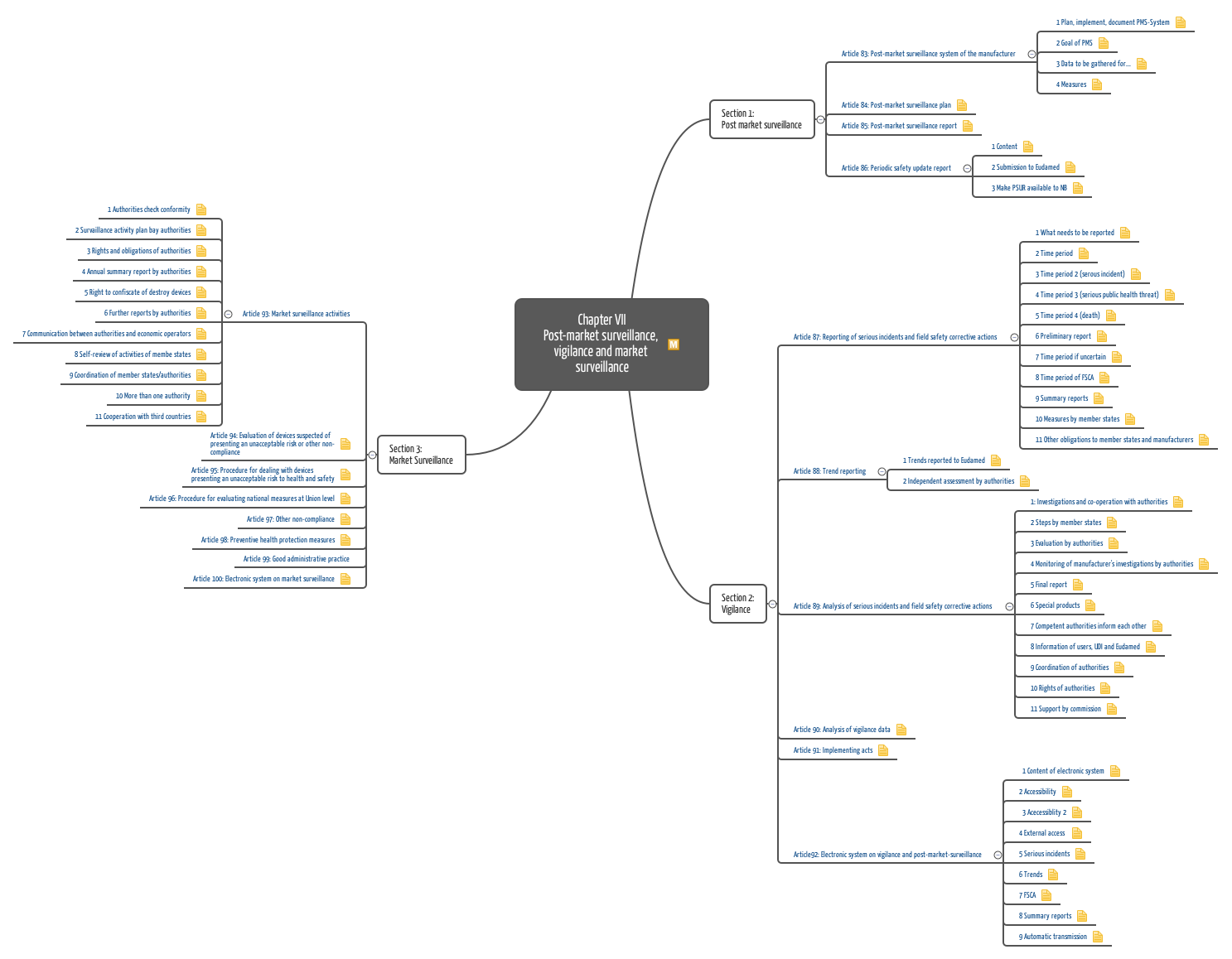
Post Market Surveillance

Post Market Surveillance: A Concise Overview of Requirements

Get Our Image of Medical Device Marketing Plan Template Marketing

Mdr Post Market Surveillance Plan Template

Performance evaluation post market surveillance and the IVDR

PMS Plan Download a Free PMS Plan Template
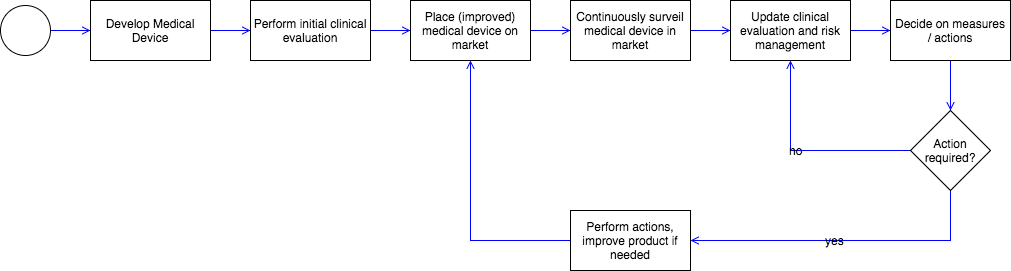
Post Market Surveillance
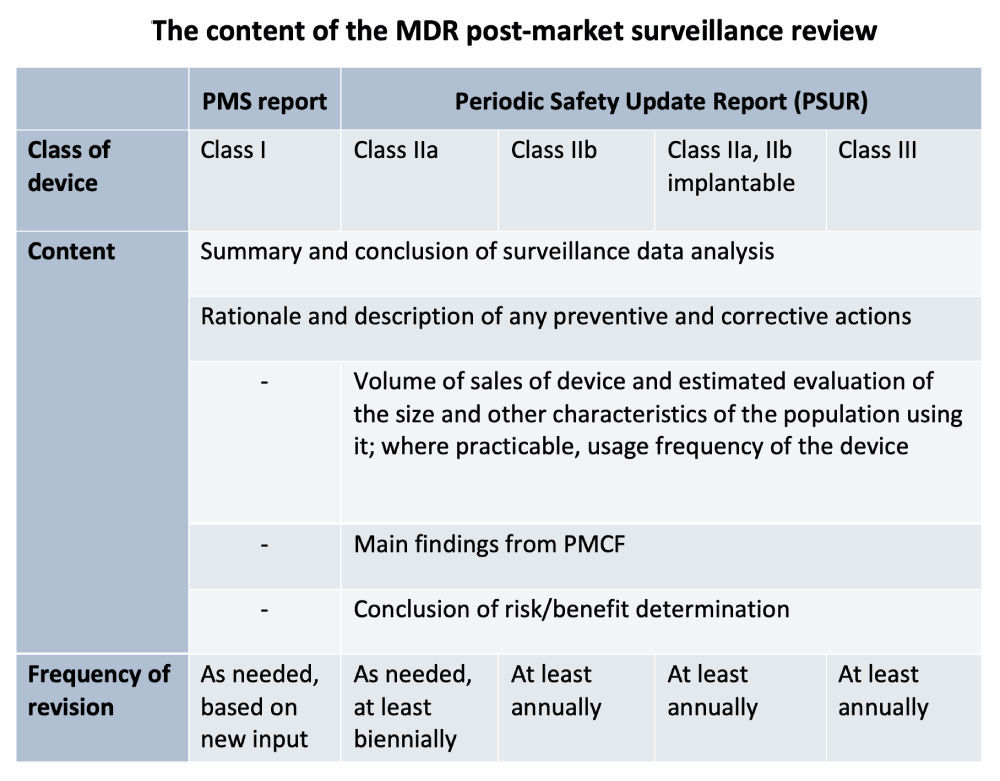
Post market surveillance requirements according to the EU MDR

Post Market Surveillance Plan Template 43 Koleksi Gambar
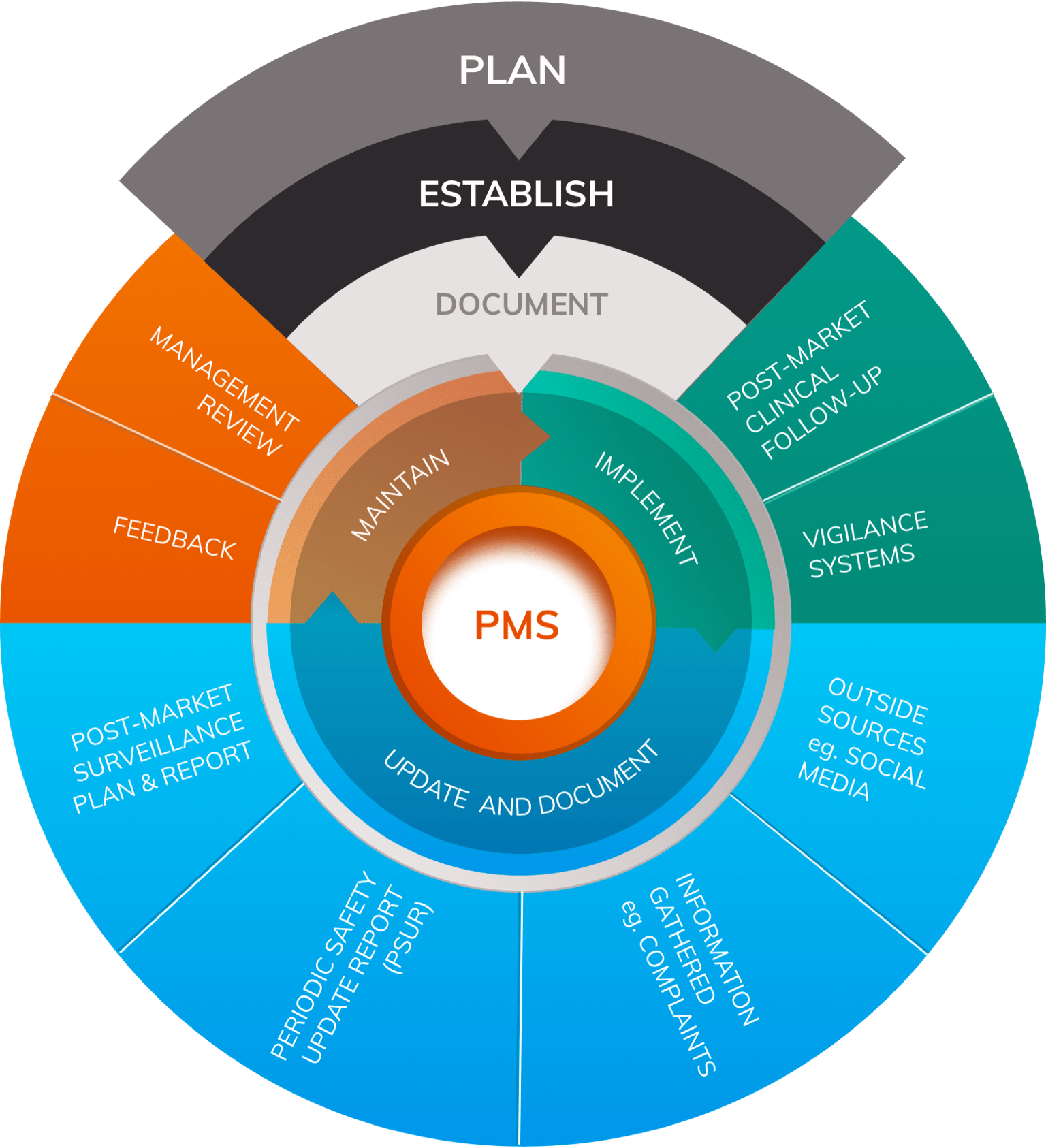
Clinical Evaluation for the EU MDR